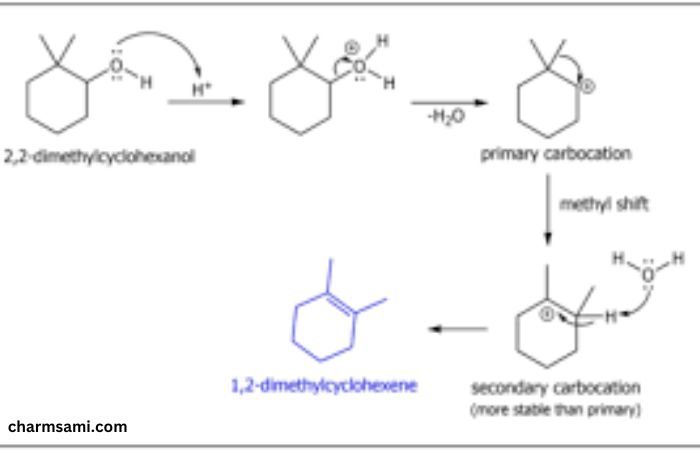
2 2-dimethylcyclohexanol with deuterium is a specialized organic compound characterized by a cyclohexane ring substituted with two methyl groups and a hydroxyl group. This compound is of significant interest in various chemical and pharmaceutical research areas due to its unique structural features and reactivity. When this compound is labeled with deuterium, an isotope of hydrogen, its properties and applications expand further, particularly in the fields of analytical chemistry and drug development.
Importance and Applications in Chemistry
The importance of 2 2-dimethylcyclohexanol with deuterium lies in its versatility as a building block in organic synthesis. It serves as an intermediate in the production of more complex molecules and is particularly useful in the study of reaction mechanisms. The introduction of deuterium into this compound enhances its value in scientific research, providing a non-radioactive label that is detectable through various spectroscopic methods.
Molecular Formula and Structure
2,2-Dimethylcyclohexanol has the molecular formula C8H16O. Its structure features a six-membered cyclohexane ring, with two methyl groups attached to the second carbon atom and a hydroxyl group (-OH) attached to the same carbon. The deuterium-labeled variant of this compound includes one or more hydrogen atoms replaced by deuterium (D), giving it the molecular formula C8H15DO.
Physical and Chemical Properties
This compound typically exists as a colorless liquid with a boiling point of around 167-168°C. Its chemical properties are largely defined by the presence of the hydroxyl group, which makes it an alcohol, capable of forming hydrogen bonds. The introduction of deuterium affects the compound’s NMR spectrum, making it easier to study its behavior in various chemical environments.
Synthesis of 2,2-Dimethylcyclohexanol
The synthesis of 2 2-dimethylcyclohexanol with deuterium involves several steps, starting with the preparation of the cyclohexane ring and subsequent methylation and hydroxylation. When deuterium is introduced, special care is taken to replace hydrogen atoms selectively, often using deuterium oxide (D2O) or deuterated reagents.
Reagents and Conditions Required
Key reagents in the synthesis include cyclohexanone, methylating agents such as methyl iodide, and a reducing agent like sodium borohydride. The reaction is typically carried out under anhydrous conditions to prevent unwanted side reactions.
Step-by-Step Synthesis Guide
- Preparation of Cyclohexanone: Begin by oxidizing cyclohexanol to cyclohexanone.
- Methylation: Introduce the methyl groups using a methylating agent.
- Reduction: Reduce the ketone group to an alcohol using sodium borohydride.
- Deuterium Labeling: Incorporate deuterium by reacting the compound with D2O under controlled conditions.
Role of Deuterium in 2,2-Dimethylcyclohexanol
Deuterium, a stable isotope of hydrogen, contains one proton and one neutron in its nucleus, giving it a greater mass compared to ordinary hydrogen. This slight difference in mass has profound effects on the behavior of molecules in which deuterium is incorporated.
Benefits of Deuterium Labeling
Labeling 2 2-dimethylcyclohexanol with deuterium is beneficial for various reasons. It allows for precise tracking of the compound in complex mixtures, aids in the study of reaction mechanisms, and improves the accuracy of analytical techniques like Nuclear Magnetic Resonance (NMR) spectroscopy.
Impact on Chemical Reactions and Stability
Deuterium labeling can also affect the reactivity and stability of the compound. The bond strength between carbon and deuterium is slightly stronger than that between carbon and hydrogen, which can slow down certain reaction rates. This isotope effect is particularly useful in studying the kinetics of chemical reactions.
In organic synthesis, 2 2-dimethylcyclohexanol with deuterium serves as a precursor to various other compounds, including pharmaceuticals and fine chemicals. Its deuterium-labeled variant is especially valuable in mechanistic studies, where tracking the movement and transformation of atoms within a molecule is crucial.
Applications in Pharmaceutical Research
In pharmaceutical research, deuterium-labeled 2 2-dimethylcyclohexanol with deuterium is used to trace the metabolic pathways of drugs. Its unique labeling allows researchers to follow the absorption, distribution, metabolism, and excretion (ADME) of drug candidates more accurately.
Deuterium-Labeled Compounds in NMR Spectroscopy
NMR spectroscopy is a powerful tool in chemistry, and deuterium labeling enhances its utility. The presence of deuterium in 2,2-Dimethylcyclohexanol alters the magnetic environment, providing clearer and more informative spectra that are essential for elucidating molecular structures.
Identifying and Quantifying 2,2-Dimethylcyclohexanol
Analytical methods for 2 2-dimethylcyclohexanol with deuterium, particularly its deuterium-labeled form, involve a combination of spectroscopic and chromatographic techniques. Accurate identification and quantification are crucial in both research and industrial applications.
Nuclear Magnetic Resonance (NMR) and Infrared (IR) spectroscopy are the primary techniques used to analyze this compound. NMR is particularly useful in observing the effects of deuterium labeling, while IR provides insights into the functional groups present in the molecule.
Chromatographic Techniques (GC, HPLC)
Gas Chromatography (GC) and High-Performance Liquid Chromatography (HPLC) are used to separate and quantify 2,2-Dimethylcyclohexanol from complex mixtures. These techniques are essential in quality control and during the synthesis process to ensure the purity of the final product.
When working with 2,2-Dimethylcyclohexanol, especially in its deuterium-labeled form, it’s essential to follow standard laboratory safety protocols. This includes wearing protective clothing, using fume hoods, and avoiding direct contact with the compound.
Proper Storage Conditions
Store 2,2-Dimethylcyclohexanol in a cool, dry place, away from light and moisture. Deuterium-labeled compounds should be stored in sealed containers to prevent isotopic exchange with atmospheric moisture. Care must be taken when handling deuterium-labeled compounds to avoid contamination with regular hydrogen. This ensures the integrity of the isotopic label and the accuracy of subsequent analytical measurements.
Environmental Impact and Sustainability
The environmental impact of synthesizing 2 2-dimethylcyclohexanol with deuterium, particularly with deuterium, is minimal. However, it’s important to consider the sources of deuterium and the energy consumption involved in its production. Efforts are being made to improve the sustainability of chemical synthesis, including the use of greener reagents and solvents. In the case of deuterium-labeled compounds, recycling deuterium from waste products is a key focus.
Waste Management and Disposal
Proper disposal of chemical waste is crucial in minimizing the environmental impact. Deuterium-labeled waste should be treated with the same care as regular chemical waste, following guidelines for hazardous materials. The synthesis and purification of 2,2-Dimethylcyclohexanol, especially with deuterium, can be challenging. Isotopic purity must be maintained throughout the process, and specialized equipment is often required.
Limitations in Industrial Applications
While deuterium-labeled 2 2-dimethylcyclohexanol with deuterium is valuable in research, its high cost and complexity of synthesis limit its use in large-scale industrial applications. However, ongoing research may reduce these limitations in the future.
Advances in Deuterium Chemistry
Deuterium chemistry is an evolving field, with new methods for incorporating deuterium into organic molecules being developed. These advances are likely to expand the applications of deuterium-labeled compounds like 2,2-Dimethylcyclohexanol. As research continues, new applications for 2,2-Dimethylcyclohexanol are likely to emerge, particularly in areas like drug development, materials science, and environmental monitoring.
Research Trends and Innovations
Current trends in research focus on improving the efficiency and sustainability of deuterium incorporation, as well as exploring the unique properties of deuterium-labeled compounds in various chemical reactions. Compared to other cyclohexanol derivatives, 2,2-Dimethylcyclohexanol offers distinct advantages in terms of reactivity and stability. Deuterium labeling further enhances these properties, making it a preferred choice in specific research applications.
Deuterium Effects in Other Compounds
The effects of deuterium labeling are not unique to 2,2-Dimethylcyclohexanol. Other compounds also benefit from this isotopic substitution, particularly in terms of improved analytical clarity and stability. In drug development, 2,2-Dimethylcyclohexanol has been used as a model compound to study the metabolic pathways of potential pharmaceuticals. Deuterium labeling has provided valuable insights into the drug’s absorption and metabolism.
Use in Environmental Monitoring
Deuterium-labeled 2,2-Dimethylcyclohexanol has also been employed in environmental monitoring to track the fate of organic pollutants. Its unique isotopic signature allows for precise detection and analysis in complex environmental samples. This compound’s versatility and the additional benefits provided by deuterium labeling underscore its importance in modern chemistry. As research continues to advance, the applications of 2,2-Dimethylcyclohexanol are likely to expand further.
FAQs About 2 2-dimethylcyclohexanol with deuterium
What is the significance of deuterium in chemical reactions?
Deuterium, as a stable isotope of hydrogen, provides unique insights into reaction mechanisms and kinetics due to its slightly different mass compared to hydrogen.
How is 2,2-Dimethylcyclohexanol with deuterium synthesized?
It is synthesized through a series of steps involving the methylation and hydroxylation of cyclohexane, followed by the selective incorporation of deuterium.
What are the safety measures for handling deuterium-labeled compounds?
Safety measures include using protective equipment, storing the compounds in sealed containers, and avoiding direct contact to prevent contamination.
What are the common applications of 2,2-Dimethylcyclohexanol in research?
This compound is commonly used in organic synthesis, pharmaceutical research, and as a deuterium-labeled standard in NMR spectroscopy.
Can deuterium-labeled compounds be used in everyday products?
While primarily used in research, there is potential for deuterium-labeled compounds to be used in specialized products, particularly in pharmaceuticals.
Conclusion
2,2-Dimethylcyclohexanol, especially in its deuterium-labeled form, is a compound of great interest in various fields of chemistry. Its unique properties make it a valuable tool in research, particularly in the study of reaction mechanisms and the development of new pharmaceuticals.